Class Action Mental Health Lawsuits and the VA

additional funds from class action lawsuits. “
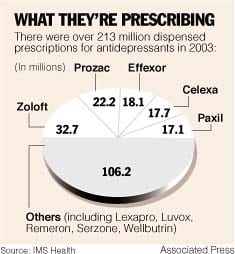
Veterans and their family members who were treated by the military or the VA HealthCare system for mental health conditions need to be aware of several class action lawsuits currently active against several psychotropic medications. The medications include Abilify, Depakote (during pregnancy), Lamictal, Lexapro (during pregnancy), Paxil, Prozac, Risperdal, Seroquel, and Zyprexa.
While these drugs work wonders for most people, for some they have caused complications that can be debilitating, disabling, and even life-threatening. If you were prescribed one of these medications by the military or the VA and any of the following symptoms manifested after starting the medication, you may be eligible for VA benefits related to residual health effects of the medication as well as be eligible for additional funds from class action lawsuits.
Mental Health Medications
- Abilify: (generic Aripiprazole) Abilify was approved to treat certain mood disorders, specifically bipolar, schizophrenia, Tourette’s disorder, and irritability associated with autistic disorder. Pharmaceutical companies encouraged prescribers to use Abilify for off label disorders such as anxiety disorders, dementia, insomnia, PTSD, and obsessive/compulsive disorders. In some people, Abilify has been linked to causing obsessive/compulsive behaviors such as gambling and drug addiction. Otsuka America Pharmaceutical and Bristol-Myers Squibb failed to inform doctors and patients of the links between Abilify and compulsive behaviors, they hid evidence from the FDA and convinced doctors to prescribe Abilify off label (for conditions not approved by the FDA).
- Depakote and Lexapro: (generic divalproex sodium and escitalopram) Both drugs have been linked to birth defects when taken by pregnant women and to increased suicidal behaviors. Users of Depakote had a 12-fold increased chance of having a child with spina-bifida and there are also links to lower cognitive function. Lexapro (an SSRI) is linked to the birth defects omphalocele, craniosynostosis, and anencephaly.
- Lamictal: (generic Lamotrigine) Approved to treat epilepsy and bipolar disorder, Lamictal has been linked to aseptic meningitis, suicidal thoughts, birth defects, Stevens-Johnson syndrome, and Toxic Epidermal Necrolysis (also known as Lamictal rash). Glaxo-SmithKline failed to warn of the rare side effects and utilized a very aggressive marketing campaign to increase sales of its drug. The resulting settlement of $3 billion was one of the largest in history.
- Paxil: (generic Paroxetine) An SSRI also distributed by Glaxo-SmithKline, Paxil was promoted as non-habit forming when the company knew of severe withdrawal symptoms including anxiety, insomnia, irritability, nausea, and fatigue. Paxil is also linked to birth defects (especially heart defects when taken in the first trimester) and suicidal thoughts, with a specifically increased risk among 18-24 year-olds.
- Prozac: (generic Fluoxetine) Another SSRI, distributed by Eli Lilly, Prozac is linked to birth defects and suicidal thoughts, especially among younger patients. Eli Lilly failed to report potential side effects for almost a decade. While studies have linked birth defects to Prozac, the FDA has specifically connected persistent pulmonary hypertension of newborns to Prozac taken during pregnancy.
- Risperdal: (generic Risperidone) An antipsychotic approved for the treatment of schizophrenia, it has been linked to the abnormal growth of breast tissue in young men. Johnson & Johnson was aware of the risks and failed to increase warning labels despite requests by prescribers and was also accused of manipulating dated and withholding key data from the FDA to gain approval of the widely used drug for use with adolescents. Makers Johnson & Johnson were also cited for failing to explore and explain what appeared to be an excessive number of deaths among elderly patients. Risperdal is limited to use for schizophrenia, bipolar disorder, and irritability associated with autistic disorder.
- Seroquel: (generic Quetiapine Fumarate) This medication was approved by the FDA for use in the treatment of schizophrenia in adults and adolescents 13 years old and up and bipolar disorder in adults and adolescents 10 years old and up but AstraZeneca marketed for the treatment of dementia. The FDA has determined that Seroquel has increased the risk of death among dementia patients due to various side effects, mainly due to cardiovascular or infectious disease issues. Seroquel is also related to Tardive Dyskinesia (TDK), which is an irreversible condition characterized by involuntary facial movements and Neuroleptic Malignant Syndrome (NMS) which is a potentially fatal disorder that causes hyperpyrexia, muscle rigidity, altered mental status, and irregular heartbeat or blood pressure.
- Zyprexa: (generic Olanzapine) Eli Lilly was approved to market Zyprexa to sufferers of schizophrenia and bipolar disorder, but was marketed as effective for people with other mental conditions including Alzheimer’s dementia. Zyprexa has been linked to diabetes, excessive weight gain, pancreatitis, TDK, and NMS.
Are You Using These Medications?
If you have been safely using any of the above medications and are not experiencing any of the symptoms listed, please review any concerns with your treating providers but do not discontinue your medications without speaking to your provider.
If you have any of the conditions mentioned above and have taken the medication associated with it, please stay tuned to our page for future updates.